Write the appropriate symbol for each of the following isotopes – Delving into the realm of isotopes, this guide embarks on a journey to decipher the intricacies of their symbolic representation. We will explore the conventions and applications of isotope notation, unraveling the secrets behind the unique identifiers assigned to these atomic variations.
Isotopes, atoms with varying neutron counts, play a pivotal role in diverse scientific fields, from medicine to archaeology. Understanding their symbolic representation is crucial for comprehending their properties and applications.
Isotopes: Identification, Notation, and Applications

Isotopes are variations of an element with the same number of protons but different numbers of neutrons. This difference in neutron count affects the mass of the isotope, giving rise to different isotopic forms of the same element.
Identifying and Writing Isotope Symbols
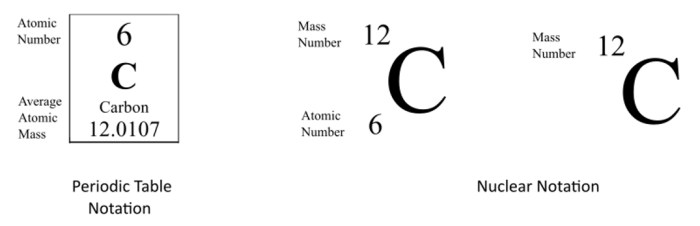
Isotope symbols are written using a combination of the element symbol and superscripts and subscripts to indicate the number of protons and neutrons in the nucleus.
- Mass number:Superscript on the left of the element symbol, representing the total number of protons and neutrons in the nucleus.
- Element symbol:The chemical symbol of the element, indicating the number of protons.
- Atomic number:Subscript on the left of the element symbol, indicating the number of protons in the nucleus (which is the same for all isotopes of an element).
Examples of Isotope Symbols, Write the appropriate symbol for each of the following isotopes
- Carbon-12: 12C
- Hydrogen-2: 2H
- Oxygen-16: 16O
- Nitrogen-14: 14N
- Chlorine-35: 35Cl
Applications of Isotopes
Isotopes have a wide range of applications in various fields:
- Medicine:Radioactive isotopes are used as tracers to study metabolic processes and diagnose diseases.
- Environmental science:Isotopes are used for carbon dating, which determines the age of organic materials.
- Archaeology:Isotopes are used for potassium-argon dating, which determines the age of rocks and minerals.
Questions Often Asked: Write The Appropriate Symbol For Each Of The Following Isotopes
What is the significance of isotope symbols?
Isotope symbols provide a concise and unambiguous way to identify and distinguish isotopes of the same element, facilitating scientific communication and data interpretation.
How are isotope symbols constructed?
Isotope symbols consist of the element symbol followed by a superscript mass number (indicating the total number of protons and neutrons) and a subscript atomic number (indicating the number of protons).
What are some examples of isotope symbols?
Carbon-12: 12C, Hydrogen-2: 2H, Oxygen-16: 16O, Nitrogen-14: 14N, Chlorine-35: 35Cl